eTMS FDA Trial for PTSD
Wright State University and Wave Neuroscience are conducting a clinical trial in Dayton, Ohio on the safety and efficacy of Electroencephalogram (EEG) Personalized Transcranial Magnetic Stimulation (eTMS) for Veterans, Law Enforcement and First Responders.

Are you eligible for this study?
- Applicant must be a military Veteran, or current/former Law Enforcement or First Responder (EMT, Firefighter, etc.)
- 22-65 years of age
- Experiencing symptoms of PTSD (No formal diagnosis is required to qualify)
- No uncontrolled psychological or medial disorders or alcohol abuse
- Not currently using neurostimulation devices
- No iron-containing metal implants near the head or medical devices that could affected by a magnetic field
- No cerebral damage, such as a stroke
Symptoms of Post-Traumatic Stress Disorder (PTSD) may include:
- Thoughts, memories, or feelings that impair daily function
- Sleep disturbance
- Dizziness
- Forgetfulness
- Fatigue
- Feeling depressed or sad


What is eTMS?
The investigative study treatment, eTMS, is a personalized form of TMS that uses the participant’s EEG signal to assign the frequency of stimulation.
Personalization of TMS is investigative. The purpose of this study is to investigate whether eTMS improves symptoms associated with PTSD.
TMS is FDA-cleared for use in Major Depressive Disorder, Obsessive-Compulsive Disorder, and Nicotine Cessation.
What does this study
entail?
3 Data Collection Visits
- Baseline recording
- Before first treatment
- After last treatment
20 Study Treatment Visits
- Study treatment visits over a 4 to 5 week period.

The eTMS Process
Step 1:
Brainwave Recording
10-Minute Scan
Eyes closed in a dark room to measure your brainwaves to give us an understanding of tour brain’s activity.

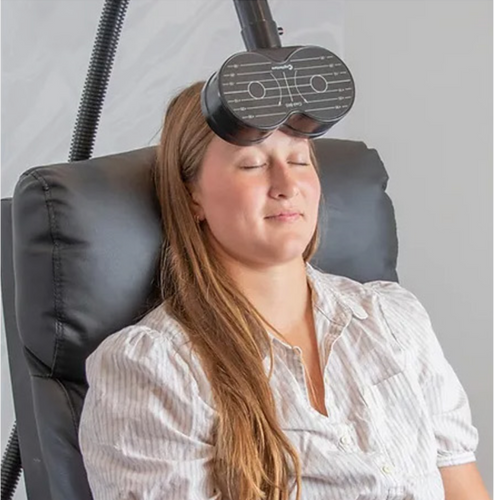
Step 2:
Investigational
Treatment
Neuromodulation
Personalized Magnetic Stimulation based on the insights gathered from you Brainwave Recording.

Non-Invasive
Non-Pharmacological
FDA Cleared Recording Equipment

Wave Neuroscience is the global leader in personalized Braincare™
275,000
Treatments Delivered
55,000+
EEG Database
14
Clinical Trials Completed
Meet Our Team
Dr. Bill Phillips
Bill has 25 years’ experience in medical device design, development, and clinical trials. HE received his PhD from the University of Southern California, specializing in brain imaging. His research has been focused on finding ways to influence brain activity in the treatment of mental disorders. He is a founder of Wave Neuroscience and currently leads their clinical research effort.


Dr. Matt Sherwood
Matt has over 10 years’ experience in defense-related brain imaging and neuromodulation research. He earned his bachelor’s and Master’s degrees in biomedical engineering, his PhD in Engineering at Wright State University focused on neurofeedback, a form of neuromodulation, for the treatment of tinnitus. HE currently is the founding Executive Director of the center for Neuroimaging and Neuro-Evaluation of Cognitive Technologies at Wright State University where he leads a diverse portfolio of neuromodulation and neuroimaging research.


